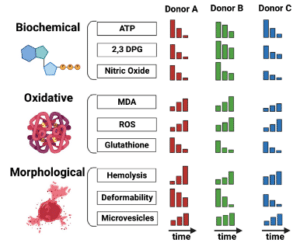
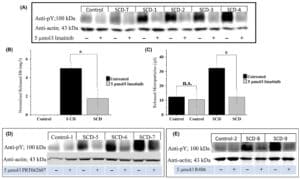
- Chronic upregulation of P-selectin on blood cells and the endothelium leads to abnormal red blood cell (RBC) adhesion to endothelial cells, significantly contributing to vaso-occlusive crises (VOCs), which are a major cause of morbidity and mortality in patients with sickle cell disease (SCD). Crizanlizumab (a.k.a. SEG101) is a humanized anti-P-selectin monoclonal antibody and has recently been approved by the Food and Drug Administration to reduce the frequency of VOCs in SCD patients.
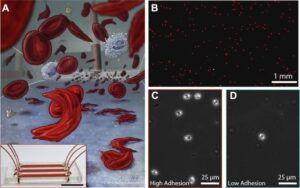
- There is currently no existing feasible clinical in vitro model for monitoring the patient-specific effects of Crizanlizumab on cellular adhesion, which we would argue is largely due to the lack of a universally accepted, standardized physiologic flow-based adhesion assay with which to measure blood cell adhesion to human endothelial cells. Utilizing such an in vitro assay would greatly help visualize cellular adhesion before and after therapeutic interventions and may reveal patient-specific responses to combination therapies.
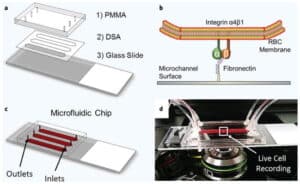
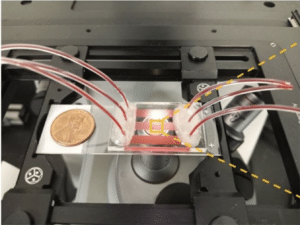
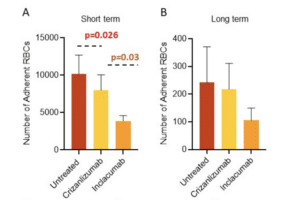
- To this end, we have developed a standardized endothelialized microfluidic platform: Endothelium-on-a-chip, for in vitro assessment of the effect of Crizanlizumab on patient-specific RBC adhesion to heme-activated human endothelial cells. We have previously shown that RBC adhesion to heme activated endothelial cells may correlate with a more severe disease phenotype.