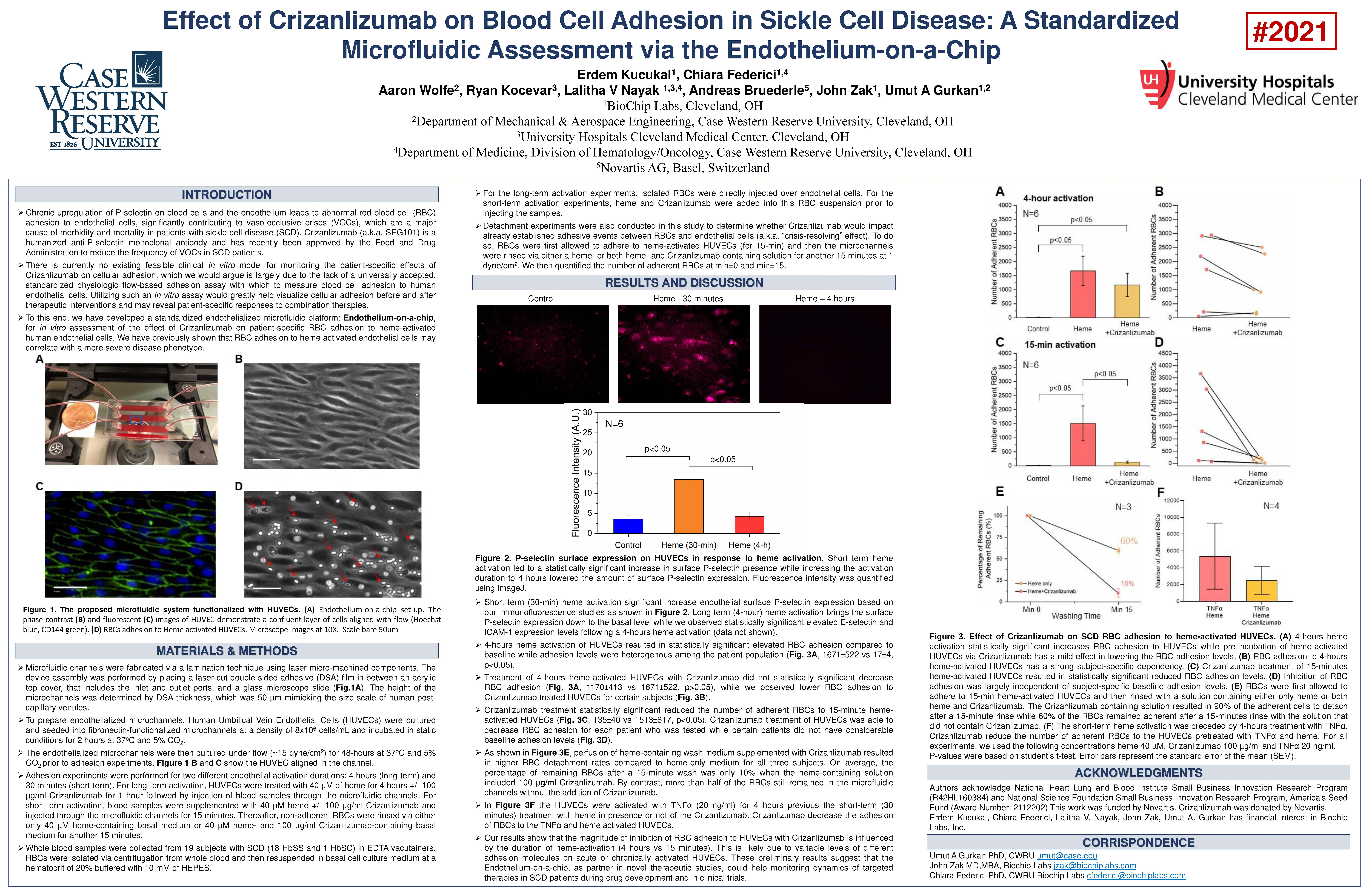
Crizanlizumab is a recent FDA approved humanized IgG2 anti-P-selectin antibody to decrease the frequency of vaso-occlusive crisis VOCs in Sickle cell disease (SCD) patients. Inclacumab is a full human IgG4 monoclonal antibody that selectively targets P-selectin. Anti-cell adhesion effects of Inclacumab were first reported in patients with cardiovascular disease. Two phase 3 clinical trials are evaluating the efficacy of Inclacumab in SCD patients to reduce VOCs (NCT04935879 and NCT04927247)