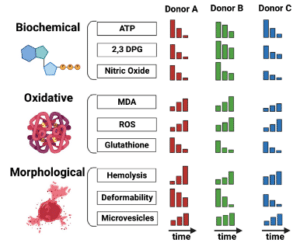
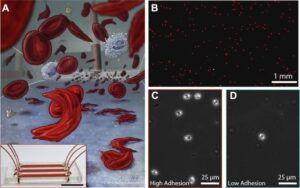
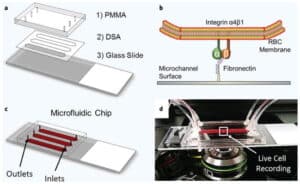
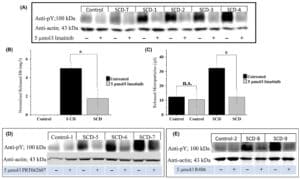
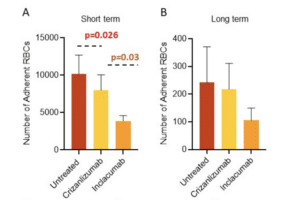
In sickle cell disease (SCD), ‘disease severity’ associates with increased RBC adhesion to quiescent endothelium, but the impact on activated endothelium is not known. Increased concentrations of free heme result from intravascular hemolysis in SCD. Heme is essential for aerobic metabolism and plays an important role in numerous biological processes. Excess free heme induces reactive oxygen species generation and endothelial activation, which are associated with cardiovascular disorders including atherosclerosis, hypertension, and thrombosis. Here, we utilized an endothelialized microfluidic platform (Endothelium-on-a-chip) to assess adhesion of sickle hemoglobin-containing red blood cells (HbSRBCs), from adults with homozygous SCD, to heme activated human endothelial cells (EC) in vitro. Confluent EC monolayers in microchannels were treated with pathophysiologically relevant levels of heme in order to simulate the highly hemolytic intravascular milieu seen in SCD. RBC adhesion to heme-activated ECs varied from subject to subject, and was associated with plasma markers of hemolysis (LDH) and reticulocytosis, thereby linking those RBCs that are most likely to adhere with those that are most likely to hemolyze. These results re-emphasize the critical contribution made by heterogeneous adhesive HbSRBCs to the pathophysiology of SCD. We found that adhesion of HbSRBCs to heme-activated ECs varied amongst individuals in the study population, and associated with biomarkers of hemolysis and inflammation, age, and a recent history of transfusion. Importantly, the microfluidic approach described herein holds promise as a clinically feasible Endothelium-on-a-chipplat form with which to study complex heterocellular adhesive interactions in SCD.